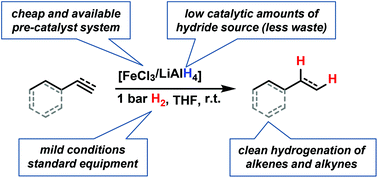
The scope and mechanism of a practical protocol for the iron-catalyzed hydrogenation of alkenes and alkynes at 1 bar H2 pressure were studied. The catalyst is formed from cheap chemicals (5 mol% FeCl3–LiAlH4, THF). A homogeneous mechanism operates at early stages of the reaction while active nanoparticles form upon ageing of the catalyst solution.
Iron-catalyzed olefin hydrogenation at 1 bar H2 with a FeCl3–LiAlH4catalyst
DOI: 10.1039/C4GC02368D

Prof. Dr. Axel Jacobi von Wangelin
Research Group , Axel Jacobi von Wangelin
Building CH, 13.1.82
Phone +49 (0)941 943-4802
Fax +49 (0)941 943-4617
http://www.uni-regensburg.de/index.html.en
Professor of Organic Chemistry (2011), Heisenberg Group Leader (Cologne, 2011) Emmy Noether Group Leader (Cologne, 2005-2010), DAAD Postdoc (Stanford, B. M. Trost, 2003-2004), Postdoc (Cardiff, K. J. Cavell, 2003), Visiting Scientist (Degussa, 2002-2003), Ph.D. (Rostock, M. Beller, 2002), Visiting Scholar (Utah, J. A. Gladysz, 1999), M.Sc. (Erlangen, J. A. Gladysz, 1998), B.Sc. (Erlangen, 1994)
Awards: ORCHEM Award of the Liebig Association (2012), Heisenberg Fellow of the DFG (2011), Science Award of the Industrieclub (2009), Thieme Journal Award (2007), Emmy Noether Fellow of the DFG (2005-2010), DAAD Fellow (2003-2004), Joachim Jungius Dissertation Award (2002)
| Most cited publications: |
| 1. Coming of Age: Sustainable Iron-Catalyzed Cross-Coupling Reactions Times Cited: 285 (Web of Science Core Collection®) |
| 2. Multicomponent coupling reactions for organic synthesis: Chemoselective reactions with amide-aldehyde mixtures Times Cited: 173 (Web of Science Core Collection®) |
| 3. Dinuclear Zn-catalyzed asymmetric alkynylation of unsaturated aldehydes Times Cited: 155 (Web of Science Core Collection®) |


 University of Regensburg
University of Regensburg