
The development of Green Chemistry inevitably involves the development of green reagents. In this review, we highlight that Cp2TiCl is a reagent widely used in radical and organometallic chemistry, which shows, if not all, at least some of the 12 principles summarized for Green Chemistry, such as waste minimization, catalysis, safer solvents, toxicity, energy efficiency, and atom economy. Also, this complex has proved to be an ideal reagent for green C–C and C–O bond forming reactions, green reduction, isomerization, and deoxygenation reactions of several functional organic groups as we demonstrate throughout the review.
Cp2TiCl: An Ideal Reagent for Green Chemistry?
http://pubs.acs.org/doi/10.1021/acs.oprd.7b00098
Synthesis and Properties of Cp2TiCl

Although Cp2TiCl is not a renewable feedstock, it is important to say that this SET is a good alternative to them because it is obtained from nonhazardous materials and also because titanium is one of the most abundant and safe transition metals on Earth
///////////Cp2TiCl
Titanocene dichloride
- Molecular FormulaC10H10Cl2Ti
- Average mass248.959 Da
 |
|
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
Dichloridobis(η5-cyclopentadienyl)titanium
|
|
| Other names
titanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV)
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.669 |
|
PubChem CID
|
|
| RTECS number | XR2050000 |
| Properties | |
| C10H10Cl2Ti | |
| Molar mass | 248.96 g/mol |
| Appearance | bright red solid |
| Density | 1.60 g/cm3, solid |
| Melting point | 289 °C (552 °F; 562 K) |
| sl. sol. with hydrolysis | |
| Structure | |
| Triclinic | |
| Dist. tetrahedral | |
| Hazards | |
| R-phrases(outdated) | R37, R38 |
| S-phrases(outdated) | S36 |
| NFPA 704 | |
| Related compounds | |
|
Related compounds
|
Ferrocene Zirconocene dichloride Hafnocene dichloride Vanadocene dichloride Niobocene dichloride Tantalocene dichloride Molybdocene dichloride Tungstenocene dichloride TiCl4 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
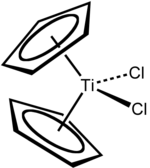
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air.[1]Cp2TiCl2 does not adopt the typical “sandwich” structure like ferrocene due to the 4 ligands around the metal centre, but rather takes on a distorted tetrahedral shape.[2] It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.[3]
Preparation
The standard preparations of Cp2TiCl2 start with titanium tetrachloride. The original synthesis by Geoffrey Wilkinson and Birmingham uses sodium cyclopentadienide[4] is still commonly used:
- 2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl
The reaction is conducted in THF. Workup sometimes washing with hydrochloric acid to convert hydrolysis derivatives to the dichloride. Recrystallization from toluene forms acicular crystals.
Cp2TiCl2 can also be prepared by using freshly distilled cyclopentadiene rather than its sodium derivative:
- 2 C5H6 + TiCl4 → (C5H5)2TiCl2 + 2 HCl
This reaction is conducted under a nitrogen atmosphere and by using THF as solvent. The product is purified by soxhlet extractionusing toluene as solvent.[5]
The complex is pseudotetrahedral. Each of the two Cp rings are attached as η5 ligands.
Applications in organic synthesis
Cp2TiCl2 is a generally useful reagent that effectively behaves as a source of Cp2Ti2+. A large range of nucleophiles will displace chloride. Examples:
- The Petasis reagent, Cp2Ti(CH3)2, is prepared from the action of methylmagnesium chloride[6] or methyllithium[7] on Cp2TiCl2. This reagent is useful for the conversion of esters into vinyl ethers.
- The Tebbe reagent Cp2TiCl(CH2)Al(CH3)2, arises by the action of 2 equivalents Al(CH3)3 on Cp2TiCl2.[8][9]
Application in preparing sulfur allotropes[edit]
Titanocene dichloride is used to prepare titanocene pentasulfide, a precursor to unusual alloptropes of sulfur:
- Li2S5 + (C5H5)2TiCl2 → (C5H5)2TiS5 + LiCl
Structure of pentasulfur-The resulting pentasulfur-titanocene complex is allowed to react with polysulfur dichloride to give the desired cyclosulfur of in the series:[10]
Reactions
Cp2TiCl2 undergoes anion exchange reactions, e.g. to give the pseudohalides. With NaSH and with polysulfide salts, one obtains the sulfido derivatives Cp2Ti(SH)2 and Cp2TiS5.
One Cp ligand can be removed from Cp2TiCl2 to give tetrahedral CpTiCl3. This conversion can be effected with TiCl4 or by reaction with SOCl2.[11]
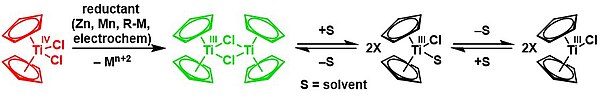
Reduction with zinc gives the dimer of bis(cyclopentadienyl)titanium(III) chloride in a solvent-mediated chemical equilibrium:[12][13]
Ti(II) derivatives
Cp2TiCl2 is a precursor to many TiII derivatives, though titanoce itself, TiCp2, is so highly reactive that it rearranges into a TiIII hydride dimer and has been the subject of much investigation.[14][15] This dimer can be trapped by conducting the reduction of titanocene dichloride in the presence of ligands; in the presence of benzene, a fulvalene complex, μ(η5:η5-fulvalene)-di-(μ-hydrido)-bis(η5-cyclopentadienyltitanium), can be prepared and the resulting solvate structurally characterised by X-ray crystallography.[16] The same compound had been reported earlier by a lithium aluminium hydride reduction[17] and sodium amalgam reduction[18] of titanocene dichloride, and studied by 1H NMR[19] prior to its definitive characterisation.[14][15]
Reductions have been investigated using Grignard reagent and alkyl lithium compounds. More conveniently handled reductants include Mg, Al, or Zn. The following syntheses demonstrate some of the compounds that can be generated by reduction of titanocene dichloride in the presence of π acceptor ligands:[20]
- Cp2TiCl2 + 2 CO + Mg → Cp2Ti(CO)2 + MgCl2
- Cp2TiCl2 + 2 PR3 + Mg → Cp2Ti(PR3)2 + MgCl2
- Cp2TiCl2 + 2 Me3SiCCSiMe3 + Mg → Cp2TiMe3SiCCSiMe3 + MgCl2
With only one equivalent of reducing agent, TiIII species such as Cp2TiCl result.
Alkyne and benzyne derivatives of titanocene are well known.[21] One family of derivatives are the titanocyclopentadienes.[22]
Titanocene equivalents react with alkenyl alkynes followed by carbonylation and hydrolysis to form bicyclic cyclopentadienones, related to the Pauson–Khand reaction).[23] A similar reaction is the reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner.[24]
Reduction of titanocene dichloride in the presence of conjugated dienes such as 1,3-butadiene gives η3-allyltitanium complexes.[25] Related reactions occur with diynes. Furthermore, titanocene can catalyze C-C bond metathesis to form asymmetric diynes.[22]
Derivatives of (C5Me5)2TiCl2
Many analogues of Cp2TiCl2 are known. Prominent examples are the ring-methylated derivatives (C5H4Me)2TiCl2 and (C5Me5)2TiCl2. The ethylene complex (C5Me5)2Ti(C2H4) can be synthesised by Na reduction of (C5Me5)2TiCl2 in the presence of ethylene. The Cp compound has not been prepared. This pentamethylcyclopentadienyl (Cp*) species undergoes many reactions such as cycloadditions of alkynes.[21]
Medicinal research
Titanocene dichloride was investigated as an anticancer drug.[26] In fact, it was both the first non-platinum coordination complex and the first metallocene to undergo a clinical trial.[3]The mechanism by which it acts is not fully understood; however, it has been conjectured that its activity might be attributable to the compound’s interactions with the protein transferrin.[3][27]
References
- Jump up^ Budaver, S., ed. (1989). The Merck Index (11th ed.). Merck & Co., Inc.
- Jump up^ Clearfield, Abraham; Warner, David Keith; Saldarriaga Molina, Carlos Hermán; Ropal, Ramanathan; Bernal, Ivan; et al. (1975). “Structural Studies of (π-C5H5)2 MX2 Complexes and their Derivatives. The Structure of Bis(π-cyclopentadienyl)titanium Dichloride”. Can. J. Chem. 53 (11): 1621–1629. doi:10.1139/v75-228.
- ^ Jump up to:a b c Roat-Malone, R. M. (2007). Bioinorganic Chemistry: A Short Course (2nd ed.). John Wiley & Sons. pp. 19–20. ISBN 978-0-471-76113-6.
- Jump up^ Wilkinson, G.; Birmingham, J.G. (1954). “Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta”. J. Am. Chem. Soc. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- Jump up^ Birmingham, J. M. (1965). “Synthesis of Cyclopentadienyl Metal Compounds”. Adv. Organometal. Chem. 2: 365–413. doi:10.1016/S0065-3055(08)60082-9.
- Jump up^ Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. (2002). “Dimethyltitanocene”. Org. Synth. 79: 19.
- Jump up^ Claus, K.; Bestian, H. (1962). “Über die Einwirkung von Wasserstoff auf einige metallorganische Verbindungen und Komplexe”. Justus Liebigs Ann. Chem. 654: 8. doi:10.1002/jlac.19626540103.
- Jump up^ Herrmann, W.A. (1982). “The Methylene Bridge”. Adv. Organomet. Chem. 20: 159–263. doi:10.1016/s0065-3055(08)60522-5.
- Jump up^ Straus, D. A. (2000). “μ-Chlorobis(cyclopentadienyl)(dimethylaluminium)-μ-methylenetitanium”. Encyclopedia of Reagents for Organic Synthesis. London: John Wiley.
- Jump up^ Housecroft, Catherine E.; Sharpe, Alan G. (2008). “Chapter 16: The group 16 elements”. Inorganic Chemistry (3rd ed.). Pearson. p. 498. ISBN 978-0-13-175553-6.
- Jump up^ Chandra, K.; Sharma, R. K.; Kumar, N.; Garg, B. S. (1980). “Preparation of η5-Cyclopentadienyltitanium Trichloride and η5-Methylcyclopentadienyltitanium Trichloride”. Chem. Ind. – London. 44: 288–289.
- Jump up^ Manzer, L. E.; Mintz, E. A.; Marks, T. J. (1982). “Cyclopentadienyl Complexes of Titanium(III) and Vanadium(III)”. Inorg. Synth. 21: 84–86. doi:10.1002/9780470132524.ch18.
- Jump up^ Nugent, William A.; RajanBabu, T. V. “Transition-metal-centered radicals in organic synthesis. Titanium(III)-induced cyclization of epoxy olefins”. J. Am. Chem. Soc. 110 (25): 8561–8562. doi:10.1021/ja00233a051.
- ^ Jump up to:a b Wailes, P. C.; Coutts, R. S. P.; Weigold, H. (1974). “Titanocene”. Organometallic Chemistry of Titanium, Zirconium, and Hafnium. Organometallic Chemistry. Academic Press. pp. 229–237. ISBN 9780323156479.
- ^ Jump up to:a b c Mehrotra, R. C.; Singh, A. (2000). “4.3.6 η5-Cyclopentadienyl d-Block Metal Complexes”. Organometallic Chemistry: A Unified Approach (2nd ed.). New Delhi: New Age International Publishers. pp. 243–268. ISBN 9788122412581.
- ^ Jump up to:a b Troyanov, Sergei I.; Antropiusová, Helena; Mach, Karel (1992). “Direct proof of the molecular structure of dimeric titanocene; The X-ray structure of μ(η5:η5-fulvalene)-di-(μ-hydrido)-bis(η5-cyclopentadienyltitanium)·1.5 benzene”. J. Organomet. Chem. 427 (1): 49–55. doi:10.1016/0022-328X(92)83204-U.
- Jump up^ Antropiusová, Helena; Dosedlová, Alena; Hanuš, Vladimir; Karel, Mach (1981). “Preparation of μ-(η5:η5-Fulvalene)-di-μ-hydrido-bis(η5-cyclopentadienyltitanium) by the reduction of Cp2TiCl2 with LiAlH4 in aromatic solvents”. Transition Met. Chem. 6 (2): 90–93. doi:10.1007/BF00626113.
- Jump up^ Cuenca, Tomas; Herrmann, Wolfgang A.; Ashworth, Terence V. (1986). “Chemistry of oxophilic transition metals. 2. Novel derivatives of titanocene and zirconocene”. Organometallics. 5 (12): 2514–2517. doi:10.1021/om00143a019.
- Jump up^ Lemenovskii, D. A.; Urazowski, I. F.; Grishin, Yu K.; Roznyatovsky, V. A. (1985). “1H NMR Spectra and electronic structure of binuclear niobocene and titanocene containing fulvalene ligands”. J. Organomet. Chem. 290 (3): 301–305. doi:10.1016/0022-328X(85)87293-4.
- Jump up^ Kuester, Erik (2002). “Bis(5-2,4-cyclopentadienyl)bis(trimethylphosphine)titanium”. Encyclopedia of Reagents for Organic Synthesis. John Wiley. doi:10.1002/047084289X.rn00022.
- ^ Jump up to:a b Buchwald, S.L.; Nielsen, R.B. (1988). “Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes”. Chem. Rev. 88 (7): 1047–1058. doi:10.1021/cr00089a004.
- ^ Jump up to:a b Rosenthal, U.; et al. (2000). “What Do Titano- and Zirconocenes Do with Diynes and Polyynes?”. Chem. Rev. 33 (2): 119–129. doi:10.1021/ar9900109.
- Jump up^ Hicks, F. A.; et al. (1999). “Scope of the Intramolecular Titanocene-Catalyzed Pauson-Khand Type Reaction”. J. Am. Chem. Soc. 121 (25): 5881–5898. doi:10.1021/ja990682u.
- Jump up^ Kablaoui, N. M.; Buchwald, S. L. (1998). “Development of a Method for the Reductive Cyclization of Enones by a Titanium Catalyst”. J. Am. Chem. Soc. 118 (13): 3182–3191. doi:10.1021/ja954192n.
- Jump up^ Sato, F.; Urabe, Hirokazu; Okamoto, Sentaro (2000). “Synthesis of Organotitanium Complexes from Alkenes and Alkynes and Their Synthetic Applications”. Chem. Rev. 100(8): 2835–2886. PMID 11749307. doi:10.1021/cr990277l.
- Jump up^ WO 2004005305, Knox, R. J. & P. C. McGowan, “Metallocenes as Anti-Tumour Reagents”, issued 2004
- Jump up^ Waern, J. B.; Harris, H. H.; Lai, B.; Cai, Z.; Harding, M. M.; Dillon, C. T. (2005). “Intracellular Mapping of the Distribution of Metals Derived from the Antitumor Metallocenes”. J. Biol. Inorg. Chem. 10 (5): 443–452. doi:10.1007/s00775-005-0649-1.
Further reading
- Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. “Dimethyltitanocene Titanium, bis(η5-2,4-cyclopentadien-1-yl)dimethyl-“. Org. Synth. 79: 19.; Coll. Vol., 10.
- Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. (1983). “Cyclopentadienyldichlorotitanium(III): a free-radical-like reagent for reducing azo (N:N) multiple bonds in azo and diazo compounds”. J. Am. Chem. Soc. 105 (25): 7295–7301. doi:10.1021/ja00363a015.
- Chirik, P. J. (2010). “Group 4 Transition Metal Sandwich Complexes: Still Fresh after Almost 60 Years”. Organometallics. 29 (7): 1500–1517. doi:10.1021/om100016p.
ESR
MORE……………
Introduction
Natural product synthesis is an exigent test for newly developed methodologies. Within this context, Rajanbabu and Nugent reported a series of seminal papers about the potential role of Cp2TiCl as a new tool in organic synthesis.1 Soon afterwards, Gansäuer’s group published a collection of relevant papers where a substoichiometric version of this protocol was developed.2Those results were especially important in the development of the corresponding asymmetric reactions using chiral titanocene(III) complexes.3 After these inspiring works, titanocene(III) complexes, essentially titanocene(III) chloride (Cp2TiCl), have recently emerged as a powerful tool in organic synthesis. They are soft single-electron-transfer (SET) reagents capable of promoting different kinds of reactions, such as homolytic epoxide4 and oxetane5 openings, Barbier-type reactions,6 Wurtz-type reactions,7 Reformatsky-type reactions,8 reduction reactions,9 and pinacol coupling reactions (Scheme 1).10

From a practical point of view, titanocene(III) complexes can be prepared and stored. Nevertheless, they are usually highly oxygen-sensitive compounds. Interestingly, they can be easily prepared in situ by simply stirring the corresponding titanocene(IV) precursor and manganese or zinc dust. Another key characteristic of the titanocene(III) chemistry is that whatever the reaction in which it is involved, a catalytic cycle can be closed. In that case, a titanocene(IV) regenerating agent and an electron source, such as manganese or zinc dust, are required. Although some of them have been described in the literature, only two are commonly used: the simple combination of trimethylsilyl chloride and 2,4,6-collidine for aprotic reaction conditions11 and 2,4,6-collidinium hydrochloride for aqueous conditions (see Scheme 2).2
http://pubs.rsc.org/en/Content/ArticleHtml/2014/QO/c3qo00024a
Conclusions
Titanocene(III)-mediated radical processes have been applied to the synthesis of natural products of diverse nature. Beyond simple functional group interconversions, radical cyclizations, mainly from epoxides, have demonstrated their utility to yield (poly)cyclic natural skeletons, which are valuable synthons in organic synthesis. This radical approach has in many cases resulted in better yields and stereoselectivities than the cationic equivalents. In particular, the synthesis at room temperature of stereodefined terpenic skeletons without enzymatic assistance is remarkable. In this context, the main limitation of this bioinspired approach is, in fact, its extraordinary stereoselectivity, which avoids obtaining cis-fused decalins and/or substituents in axial positions. Such stereochemistry is present in many interesting natural terpenes. On the other hand, as can be seen in the first part of the review, the diverse reactivity of titanocene(III) complexes derives in some functional group incompatibilities. It is expected that in near future judicious designs of new titanocene(III) complexes can resolve this drawback. In any case, the evolution of the applications of titanocene(III) in natural product synthesis suggests that these reagents can be a matter of choice in the arsenal of a synthetic organic chemist.
/////////
//////////////











Reblogged this on New Drug Approvals.