Ali’s paper on the synthesis and characterization of vanadium amino-phenolate complexes and their reactivity in oxidation catalysis is now available online: http://dx.doi.org/10.1002/ejic.201600068 Thank you to Louise for the X-ray work and Andrew (SWASP student) for his assistance in the lab. Thanks also to the editors and referees of this paper. It is great to see […]
Month: May 2016
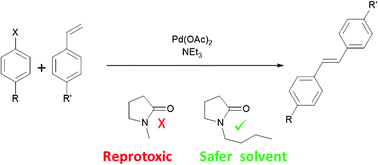
N-Butylpyrrolidinone as a dipolar aprotic solvent for organic synthesis
DOI: 10.1039/C6GC00932H, Paper
N-Butylpyrrolidinone (NBP) has been demonstrated as a suitable safer replacement solvent for N-Methylpyrrolidinone (NMP) in selected organic syntheses.
N-Butylpyrrolidinone as a dipolar aprotic solvent for organic synthesis
E-mail: andrew.hunt@york.ac.uk
DOI: 10.1039/C6GC00932H
Dipolar aprotic solvents such as N-methylpyrrolidinone (or 1-methyl-2-pyrrolidone (NMP)) are under increasing pressure from environmental regulation. NMP is a known reproductive toxin and has been placed on the EU “Substances of Very High Concern” list. Accordingly there is an urgent need for non-toxic alternatives to the dipolar aprotic solvents. N-Butylpyrrolidinone, although structurally similar to NMP, is not mutagenic or reprotoxic, yet retains many of the characteristics of a dipolar aprotic solvent. This work introduces N-butylpyrrolidinone as a new solvent for cross-coupling reactions and other syntheses typically requiring a conventional dipolar aprotic solvent.
Synthesis in mesoreactors: Ru(porphyrin)CO-catalyzed aziridination of olefins under continuous flow conditions
DOI: 10.1039/C6CY00207B, Communication
The Ru(porphyrin)CO-catalyzed addition of aryl azides to styrenes to afford N-aryl aziridines was successfully performed for the first time in mesoreactors under continuous flow conditions.
Synthesis in mesoreactors: Ru(porphyrin)CO-catalyzed aziridination of olefins under continuous flow conditions
E-mail: alessandra.puglisi@unimi.it, maurizio.benaglia@unimi.it
DOI: 10.1039/C6CY00207B
The Ru(porphyrin)CO-catalyzed addition of aryl azides to styrenes to afford N-aryl aziridines was successfully performed for the first…
View original post 37 more words
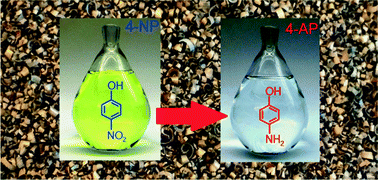
Plastically deformed Cu-based alloys as high-performance catalysts for the reduction of 4-nitrophenol
DOI: 10.1039/C6CY00734A, Paper
Plastically deformed mesoscopic structures exposed to an etching procedure are demonstrated as highly catalytic in the reduction of 4-nitrophenol.
Plastically deformed Cu-based alloys as high-performance catalysts for the reduction of 4-nitrophenol
Plastically deformed Cu-based alloys as high-performance catalysts for the reduction of 4-nitrophenol
E-mail: neretina@temple.edu
DOI: 10.1039/C6CY00734A, http://pubs.rsc.org/en/Content/ArticleLanding/2016/CY/C6CY00734A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2Fcy+%28RSC+-+Catalysis+Science+%26+Technology+latest+articles%29#!divAbstract
The severe plastic deformation of metals leads to the formation of nanotextured surfaces as well as the retention…
View original post 169 more words