Microbial cyclosophoraose as a catalyst for the synthesis of diversified indolyl 4H-chromenes via one-pot three component reactions in water
Green Chem., 2016, Advance Article DOI: 10.1039/C6GC00137H, Paper Someshwar D. Dindulkar, Daham Jeong, Eunae Cho, Dongjin Kim, Seunho Jung A novel biosourced saccharide catalyst, microbial cyclosophoraose, a cyclic [small beta]-(1,2) glucan, was used for the synthesis of indolyl 4H-chromenes via a one pot three-component Knoevenagel-Michael addition-cyclization reaction in water under neutral conditions.
Microbial cyclosophoraose as a catalyst for the synthesis of diversified indolyl 4H-chromenes via one-pot three component reactions in water
E-mail: shjung@konkuk.ac.kr
DOI: 10.1039/C6GC00137H
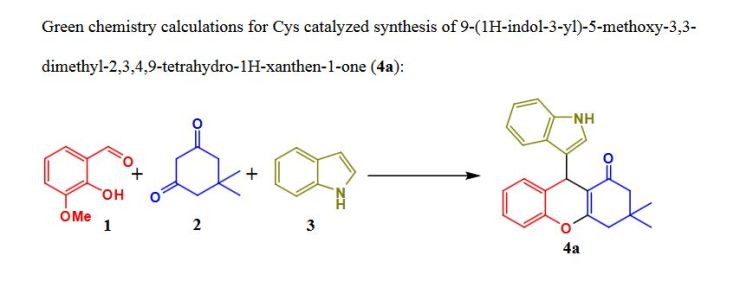
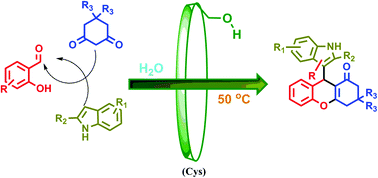
As a novel biosourced saccharide catalyst, microbial cyclosophoraose, a cyclic β-(1,2) glucan, was used for the synthesis of therapeutically important versatile indolyl 4H-chromenes via a one pot three-component Knoevenagel–Michael addition–cyclization reaction of salicylaldehyde, 1,3-cyclohexanedione/dimedone, and indoles in water under neutral conditions. A possible reaction mechanism through molecular complexation is suggested based on 2D ROESY NMR spectroscopic analysis. Moreover, green chemistry metric calculations were carried out for a model reaction, indicating the satisfactory greener approach of this method, with a low E-factor (0.18) and high atom economy (AE = 91.20%). The key features of this protocol are based on two critical factors where the first is to use a novel eco-friendly supramolecular carbohydrate catalyst and the second is its fine green properties such as compatibility with various substituted reactants, recyclability of the catalyst, chromatography-free purification, high product selectivity, and clean conversion with moderate to excellent yields in an aqueous medium.




////////Microbial cyclosophoraose, catalyst, synthesis, diversified indolyl 4H-chromenes, one-pot three component reactions, water